
MYTH: YOUR GENES ARE PATENTED.
FACTS: IT IS NOT POSSIBLE TO PATENT YOUR GENES
The term “gene patent” is a misnomer, because genes as they exist in the body cannot be patented. Because a naturally-occurring gene – even a newly-discovered one – cannot be patented, patents don’t provide ownership rights over our genes, and nobody can infringe a patent by having a certain gene, or by passing it on to their children.
If genes aren’t patentable, what is?
Natural genes are not eligible for patenting, but artificial preparations of DNA molecules are, because they have new qualities that distinguish them from natural genes. Like other chemicals that are derived from nature (such as antibiotics or natural dyes), preparations of DNA molecules are patentable because they have been transformed through human intervention into something that is so different from the natural state as to qualify as new, useful, and man-made. This transformation begins with the purification and isolation of the natural DNA. But isolating and purifying is not enough. The resulting DNA preparation must have new qualities, advantages, and technical applications that allow it to be used in important new ways that are not possible with the natural gene, making it different not just in degree, but in kind. For these reasons, every nation in the developed world recognizes the patentability of such inventions.
How is a patented DNA preparation different from a natural gene?
Patented DNA preparations contain isolated DNA molecules that are stripped of everything that is necessary for the normal operation of a gene in its natural state. Such purified DNA molecules also are often reconfigured in ways that eliminate large parts of the genetic sequence of the natural gene, giving rise to DNA molecules that do not exist in nature. Such DNA molecules can be used in ways that are simply not possible with the natural gene – for example, to conduct gene transfer experiments, to make DNA vaccines, or to produce therapeutic proteins in large scale cell culture.
What else is required to make a DNA molecule patentable?
It is impermissible to patent a DNA molecule, even if isolated and purified, unless the scientist establishes the detailed biological function of the gene from which it was derived and identifies a credible, specific and substantial utility for it. Identifying a gene’s biological role and establishing a credible, specific and substantial use for the claimed DNA molecule are often harder than identifying the new gene itself, and certainly meets the standard thresholds for inventiveness. Furthermore, in every case, the inventor has to satisfy the “novelty” and “unobviousness” requirements under which the claimed DNA must be new and distinct from all preexisting scientific knowledge. Finally, the patent application must contain a detailed scientific disclosure that enables other scientists to replicate the invention.
Overall, identifying, deriving, characterizing and describing a DNA sequence in this way requires the same level of human ingenuity as synthesizing a new chemical, composing a new metal alloy, or other human creations that are commonly deemed patentable.
This post was authored by Hans Sauer, Ph.D., J.D. Deputy General Counsel for Intellectual Property at BIO and Adjunct Professor of Law, Georgetown Law.
Related Posts:
Patent Docs
IP Watch Dog
Filed under: Gene Patents | Tagged: ACLU, AMP v. Myriad Genetics, AMP v. USPTO, DNA patent requirements, DNA patents, gene patents, gene patents pros and cons, isolated DNA patents, Myriad, patenting life, patenting people | 3 Comments »
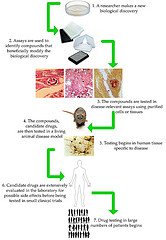 BIO is hosting a Translation Research Forum at the BIO International Convention. NIH Director Collins keynotes an event that will explore how private, public and academic sectors can leverage meaningful partnerships, highlight emerging best practices, explore risk-sharing at the clinical research stage, and explore ways to bridge the gap in funding and know-how necessary to take university research to the next level.
BIO is hosting a Translation Research Forum at the BIO International Convention. NIH Director Collins keynotes an event that will explore how private, public and academic sectors can leverage meaningful partnerships, highlight emerging best practices, explore risk-sharing at the clinical research stage, and explore ways to bridge the gap in funding and know-how necessary to take university research to the next level.






